Fossil Fuels
Fossil fuels come from the organic matter of plants, algae, and cyanobacteria that were buried, heated, and compressed under high pressure over millions of years. The process transformed the biomass of those organisms into three types of fossil fuels: oil, coal, and natural gas.
Petroleum (oil)
Thirty-seven percent of the world’s energy and 43% of the United States’s energy consumption comes from oil. Scientists and policy-makers often discuss when the world will reach peak oil production, the point at which oil production is at its greatest and then declines. It is generally thought that peak oil will be reached by the middle of the 21st Century, although making such estimates is difficult because many variables must be considered. Worldwide reserves are 1.3 trillion barrels or 45 years left at the current production level.
Environmental Impacts of Oil Extraction and Refining
Oil is usually found one to two miles (1.6 – 3.2 km) below the Earth’s surface, whether on land or ocean. Once the oil is found and extracted, it must be refined, which separates and prepares the mix of crude oil into the different types for gas, diesel, tar, and asphalt. Oil refining is one of the top sources of air pollution in the United States for volatile organic hydrocarbons, toxic emissions, and the single largest source of carcinogenic benzene. When petroleum is burned as gasoline or diesel, or to make electricity or to power boilers for heat, it produces a number of emissions that have a detrimental effect on the environment and human health:
- Carbon dioxide (CO2) is a greenhouse gas and a source of climate change.
- Sulfur dioxide (SO2) causes acid rain, which damages plants and animals that live in water and increases or causes respiratory illnesses and heart diseases, particularly in vulnerable populations like children and the elderly.
- Nitrous oxides (NOx) and Volatile Organic Carbons (VOCs) contribute to ground-level ozone, an irritant that causes lung damage.
- Particulate Matter (PM) produces hazy conditions in cities and scenic areas and combines with ozone to contribute to asthma and chronic bronchitis, especially in children and the elderly. Very small, or “fine PM,” is also thought to penetrate the respiratory system more deeply and cause emphysema and lung cancer.
- Lead can have severe health impacts, especially for children.
Other domestic sources of oil are being considered conventional resources and are being depleted. These include tar sands – moist sand and clay deposits with 1-2 percent bitumen (thick and heavy petroleum-rich in carbon and poor in hydrogen). These are removed by strip mining (see section below on coal). Another source is oil shale, which is sedimentary rock filled with organic matter that can be processed to produce liquid petroleum. Extracted by strip mining or creating subsurface mines, oil shale can be burned directly like coal or baked in hydrogen to extract liquid petroleum. However, the net energy values are low and expensive to extract and process. These resources have severe environmental impacts due to strip mining, carbon dioxide, methane, and other air pollutants similar to fossil fuels.
As the United States tries to extract more oil from its own dwindling resources, they are drilling even deeper into the earth and increasing the environmental risks. The largest United States oil spill to date began in April 2010 when an explosion occurred on the Deepwater Horizon Oil Rig, killing 11 employees and spilling nearly 200 million gallons of oil before the resulting leak could be stopped. Wildlife, ecosystems, and people’s livelihoods were adversely affected. A lot of money and huge amounts of energy were expended on immediate clean-up efforts. The long-term impacts are still not known. The National Commission on the Deepwater Horizon Oil Spill and Offshore Drilling was set up to study what went wrong.
The Global Dependence of Transportation on Oil
Two-thirds of oil consumption is devoted to transportation, fueling cars, trucks, trains, and airplanes. For the United States and most developed societies, transportation is woven into the fabric of our lives, a necessity as central to daily operations as food or shelter. The concentration of oil reserves in a few regions of the world makes much of the world dependent on imported energy for transportation. The rise in oil prices in the last decade makes dependence on imported energy for transportation an economic and an energy issue. The United States, for example, now spends upwards of $350 billion annually on imported oil, a drain of economic resources that could be used to stimulate growth, create jobs, build infrastructure, and promote social advances at home.
Coal
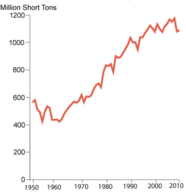
Unlike oil, coal is solid. Due to its relatively low cost and abundance, coal generates about half of the electricity consumed in the United States. Coal is the largest domestically produced source of energy. Coal production has doubled in the United States over the last sixty years (Figure 1). Current world reserves are estimated at 826,000 million tonnes, with nearly 30% of that in the United States. It is a major fuel resource that the United States controls domestically.
Coal is plentiful and inexpensive when looking only at the market cost relative to the cost of other sources of electricity, but its extraction, transportation, and use produces a multitude of environmental impacts that the market cost does not truly represent. Coal emits sulfur dioxide, nitrogen oxide, and mercury, which are linked to acid rain, smog, and health issues. Burning coal emits higher amounts of carbon dioxide per unit of energy than oil or natural gas. Coal accounted for 35% of the United States carbon dioxide emissions released into the Earth’s atmosphere in 2010. Ash generated from combustion contributes to water contamination. Some coal mining has a negative impact on ecosystems and water quality and alters landscapes and scenic views (such as with mountaintop mining).
There are also significant health effects and risks to coal miners and those living near coal mines. Traditional underground mining is risky to mine workers due to the risk of entrapment or death. Over the last 15 years, the U.S. Mine Safety and Health Administration has published the number of mine worker fatalities, varying from 18-48 per year. Twenty-nine miners died on April 6, 2010, in an explosion at the Upper Big Branch coal mine in West Virginia, contributing to the uptick in deaths between 2009 and 2010. In other countries with fewer safety regulations, accidents occur more frequently. In May 2011, for example, three people died, and 11 were trapped in a coal mine in Mexico for several days. There is also risk of getting black lung disease (pneumoconiosis). This lung disease is caused by inhaling coal dust over a long time. It causes coughing and shortness of breath. If exposure is stopped, the outcome is good. However, the complicated form may cause shortness of breath that worsens.

Mountaintop mining (MTM), while less hazardous to workers, negatively affects land resources. MTM is a surface mining practice involving the removal of mountaintops to expose coal seams and disposing of the associated mining waste in adjacent valleys. This form of mining is very damaging to the environment because it literally removes the tops of mountains, destroying the existing habitat. Additionally, the debris from MTM is dumped into valleys burying streams and other important habitats.
Natural Gas
Natural gas meets 20% of world energy needs and 25% of the United States’ needs. Natural gas is mainly composed of methane (CH4) and is a very potent greenhouse gas. There are two types of natural gas. Biogenic gas is found at shallow depths and arises from bacteria’s anaerobic decay of organic matter, like landfill gas. Thermogenic gas comes from the compression of organic matter and deep heat underground. They are found with petroleum in reservoir rocks and with coal deposits, and these fossil fuels are extracted together.
Natural gas is released into the atmosphere from coal mines, oil and gas wells, natural gas storage tanks, pipelines, and processing plants. These leaks are the source of about 25% of total U.S. methane emissions, which translates to three percent of total U.S. greenhouse gas emissions. When natural gas is produced but cannot be captured and transported economically, it is “flared” or burned at well sites, which converts it to CO2. This is considered safer and better than releasing methane into the atmosphere because CO2 is a less potent greenhouse gas than methane.
In the last few years, a new reserve of natural gas has been identified: shale resources. The United States possesses 2,552 trillion cubic feet (Tcf) (72.27 trillion cubic meters) of potential natural gas resources, with shale resources accounting for 827 Tcf (23.42 tcm). As natural gas prices increased, extracting the gas from shale has become more economical. Figure 3 shows the past and forecasted U.S. natural gas production and the various sources. The current reserves are enough to last about 110 years at the 2009 rate of U.S. consumption (about 22.8 Tcf per year -645.7 bcm per year).
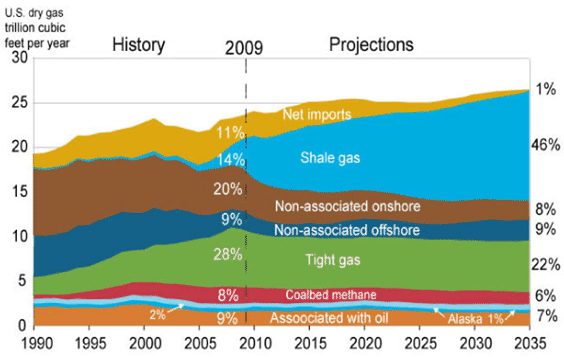
Natural gas is a preferred fossil fuel when considering its environmental impacts. Specifically, when burned, much less carbon dioxide (CO2), nitrogen oxides, and sulfur dioxide are omitted from the combustion of coal or oil. It also does not produce ash or toxic emissions.
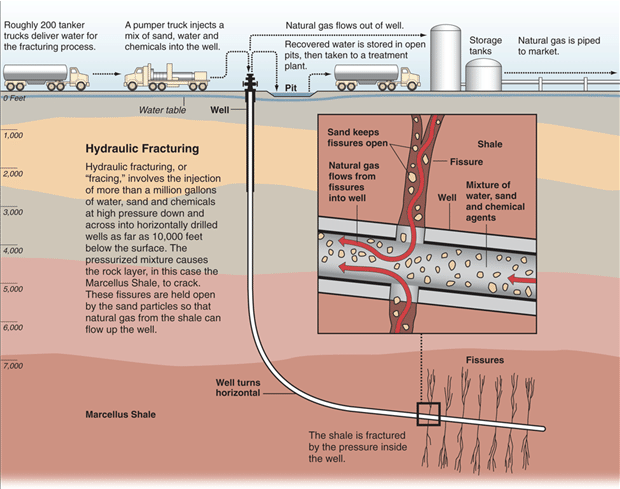
Natural gas production can result in the production of large volumes of contaminated water. This water has to be properly handled, stored, and treated so that it does not pollute land and water supplies. Shale gas extraction is more problematic than traditional sources due to a process nicknamed fracking or fracturing of wells since it requires large amounts of water (Figure 4). The technique uses high-pressure fluids to fracture the normally hard shale deposits and release gas and oil trapped inside the rock. To promote gas flow out of the rock, small particles of solids are included in the fracturing liquids to lodge in the shale cracks and keep them open after the liquids are depressurized. The considerable use of water may affect water availability for other uses in some regions, which can affect aquatic habitats. If mismanaged, hydraulic fracturing fluid can be released by spills, leaks, or various other exposure pathways. The fluid contains potentially hazardous chemicals such as hydrochloric acid, glutaraldehyde, petroleum distillate, and ethylene glycol. The risks of fracking have been highlighted in popular culture in the documentary Gasland (2010).
The raw gas from a well may contain many other compounds besides the methane being sought, including hydrogen sulfide, a very toxic gas. Natural gas with high concentrations of hydrogen sulfide is usually flared, which produces CO2, carbon monoxide, sulfur dioxide, nitrogen oxides, and many other compounds. Natural gas wells and pipelines often have engines to run equipment and compressors, which produce additional air pollutants and noise.
Contributions of Coal and Natural Gas to Electricity Generation
Currently, the US’s fossil fuels used for electricity generation are predominantly coal (44%) and natural gas (23%); petroleum accounts for approximately 1%. Coal electricity traces its origins to the early 20th Century, when it was the natural fuel for steam engines, given its abundance, high energy density, and low cost. Natural Gas is a later addition to the fossil electricity mix, arriving in significant quantities after World War II and with its greatest growth since 1990. Of the two fuels, coal emits almost twice the carbon dioxide as natural gas for the same heat output, making it a significantly greater contributor to global warming and climate change.
The Future of Natural Gas and Coal
The future development of coal and natural gas depends on the degree of public and regulatory concern for carbon emissions and the relative price and supply of the two fuels. Coal supplies are abundant in the United States, and the transportation chain from mines to power plants is well established. The primary unknown factor is the degree of public and regulatory pressure that will be placed on carbon emissions. Strong regulatory pressure on carbon emissions would favor the retirement of coal and the addition of natural gas power plants. This trend is reinforced by the recent dramatic expansion of shale gas reserves in the United States due to advances in drilling technology. Shale natural gas production increased 48% annually from 2006 – 2010, with more increases expected. Greater United States production of shale gas will gradually reduce imports and could eventually make the United States a net exporter of natural gas.
Nuclear Power
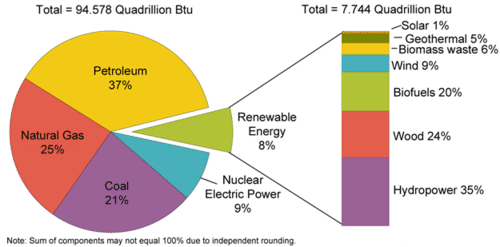
Nuclear power is energy released from the radioactive decay of elements, such as uranium, which releases large amounts of energy. Nuclear power plants produce no carbon dioxide and are often considered alternative fuels (fuels other than fossil fuels). Currently, world electricity production from nuclear power is about 19.1 trillion KWh, with the United States producing and consuming about 22% of that. Nuclear power provides about 9% of the electricity in the United States (Figure 7).
There are environmental challenges with nuclear power. Mining and refining uranium ore and making reactor fuel demands a lot of energy. Also, nuclear power plants are very expensive and require large amounts of metal, concrete, and energy to build. The main environmental challenge for nuclear power is waste, including uranium mill tailings, spent (used) reactor fuel, and other radioactive wastes. These materials have long radioactive half-lives and thus threaten human health for thousands of years. The half-life of a radioactive element is the time it takes for 50% of the material to decay radioactively. The U.S. Nuclear Regulatory Commission regulates the operation of nuclear power plants and the handling, transportation, storage, and disposal of radioactive materials to protect human health and the environment.
By volume, the waste produced from mining uranium, called uranium mill tailings, is the largest waste and contains the radioactive element radium, which decays to produce radon, a radioactive gas. High-level radioactive waste consists of used nuclear reactor fuel. This fuel is in a solid form consisting of small fuel pellets in long metal tubes and must be stored and handled with multiple containment, first cooled by water and later in special outdoor concrete or steel containers that are cooled by air. There is no long-term storage facility for this fuel in the United States.
Many other regulatory precautions govern permitting, construction, operation, and decommissioning of nuclear power plants due to risks from an uncontrolled nuclear reaction. The potential for air, water, and food contamination is high should an uncontrolled reaction occur. Even when planning for worst-case scenarios, unexpected events are always risks. For example, the March 2011 earthquake and subsequent tsunami that hit Japan resulted in reactor meltdowns at the Fukushima Daiichi Nuclear Power Station, causing massive damage to the surrounding area.
Debating Nuclear Energy
From a sustainability perspective, nuclear electricity presents an interesting dilemma. On the one hand, nuclear electricity produces no carbon emissions, a major sustainable advantage in a world facing anthropogenic climate change. On the other hand, nuclear electricity produces dangerous waste that i) must be stored out of the environment for thousands of years, ii) can produce bomb-grade plutonium and uranium that terrorists or others could divert to destroy cities and poison the environment, and iii) threatens the natural and built environment through accidental leaks of long-lived radiation. Thoughtful scientists, policymakers, and citizens must weigh the benefit of this source of carbon-free electricity against the environmental risk of storing spent fuel, the societal risk of nuclear proliferation, and the impact of accidental or deliberate release of radiation. There are very few examples of humans having the power to change the dynamics of the earth permanently. Global climate change from carbon emissions is one example, and radiation from the explosion of a sufficient number of nuclear weapons is another. Nuclear electricity touches both of these opportunities, on the positive side for reducing carbon emissions and the negative side for the risk of nuclear proliferation.
Nuclear electricity came into the energy scene remarkably quickly. Following the development of nuclear technology at the end of World War II for military ends, nuclear energy quickly acquired a new peacetime path for inexpensive electricity production. Eleven years after the end of World War II, a very short time in energy terms, the first commercial nuclear reactor produced electricity at Calder Hall in Sellafield, England. The number of nuclear reactors grew steadily to more than 400 by 1990, four years after the Chornobyl disaster in 1986 and eleven years following Three Mile Island in 1979. Since 1990, the number of operating reactors has remained approximately flat, with new construction balancing decommissioning due to public and government reluctance to proceed with nuclear electricity expansion plans.
The outcome of this debate will determine whether the world experiences a nuclear renaissance that has been in the making for several years. The global discussion has been strongly impacted by the unlikely nuclear accident in Fukushima, Japan, in March 2011. The Fukushima nuclear disaster was caused by an earthquake and tsunami that disabled the cooling system for a nuclear energy complex consisting of operating nuclear reactors and storage pools for underwater storage of spent nuclear fuel, ultimately causing a partial meltdown of some of the reactor cores and release of significant radiation. This event, 25 years after Chornobyl, reminds us that safety and public confidence are especially important in nuclear energy; without them, the expansion of nuclear energy will not happen.

